A frequent confusion in the chemical and food industries is the interchangeable use of “Phosphorus” and “Phosphate.” While they are linguistically related, they refer to distinctly different chemical entities.
The Short Answer: No.
Phosphorus (P) is a pure chemical element (Atomic Number 15), highly reactive and rarely found free in nature. Phosphate (PO₄³⁻) is a stable chemical compound (an anion) derived from phosphorus combined with oxygen. In the industrial supply chain, phosphate rock is mined to extract phosphorus, which is then processed into stable phosphates like STPP and SHMP.
1. The Chemical Distinction: Element vs. Compound
To understand the difference, we must look at the atomic level.
Phosphorus (The Element)
Phosphorus is a non-metallic element found on the periodic table with the symbol P. It is essential for life, forming the backbone of DNA and RNA, and is critical for energy transfer via ATP (Adenosine Triphosphate) in living cells.
- Reactivity: Elemental phosphorus (White Phosphorus) is extremely reactive and can spontaneously combust in air. It does not exist freely in nature.
- Forms: It exists in allotropes like White Phosphorus (toxic, industrial precursor) and Red Phosphorus (stable, used in flame retardants).
Phosphate (The Derivative)
Phosphate refers to the oxidized form of phosphorus. When one phosphorus atom bonds with four oxygen atoms in a tetrahedral arrangement, it forms the Phosphate Ion (PO₄³⁻).
- Stability: Unlike pure phosphorus, phosphates are chemically stable and safe for handling.
- Oxidation State: In orthophosphates, phosphorus is in the +5 oxidation state. This is distinct from Phosphorous Acid (H₃PO₃), where phosphorus is in the +3 state.
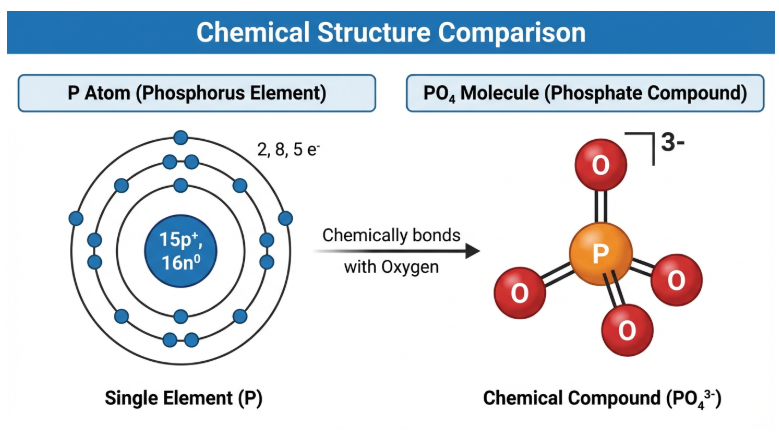
Figure 1: Structural difference between unstable Phosphorus atom and stable Phosphate ion.
2. Comparison Table: At a Glance
| Feature | Phosphorus (P) | Phosphate (PO₄) |
|---|---|---|
| Classification | Chemical Element | Chemical Compound / Ion |
| Natural State | Never free (too reactive) | Abundant in minerals (Phosphate Rock) |
| Toxicity | White P is highly toxic | Generally Safe (Food Grade E-numbers) |
| Primary Use | Raw material for chemicals | Fertilizers, Food Additives, Detergents |
⚠️ Technical Note: Unit Conversion Matters
For lab analysis and formulation, precision requires clear unit specification. Neglecting the distinction between P and PO₄ can introduce analytical errors exceeding 200%.
- Report as P: Often used in environmental or elemental analysis (mg P/L).
- Report as PO₄: Often used in water treatment and product dosing (mg PO₄/L).
Conversion Rule: 1 mg P ≈ 3.07 mg PO₄
3. The Industrial Supply Chain (From Rock to Additive)
For B2B buyers, understanding the conversion process is key to judging quality. Goway Chemical specializes in converting raw phosphate resources into high-purity additives.
- Mining: It starts with Phosphate Rock (Apatite), which is rich in calcium phosphate.
- Yellow Phosphorus Production: The rock is processed in an electric arc furnace to produce elemental Yellow Phosphorus (P4). This defines the “Thermal Process” route.
- Phosphoric Acid: Yellow P4 is burned and hydrated to create high-purity Phosphoric Acid.
- Phosphate Salts: The acid is neutralized with soda ash or caustic soda to produce salts like STPP, SHMP, and TSPP.
Note: “Thermal Process” phosphates (which we use for food grade) have much lower heavy metal content compared to “Wet Process” phosphates used for fertilizers.
4. Why the Confusion? (Medical vs. Industrial)
Confusion often arises in medical testing. A doctor might order a “Phosphorus Test” to check bone health, but the lab actually measures inorganic Phosphate levels in the blood.
However, in the chemical industry, specificity is non-negotiable. Ordering “Phosphorus” when you need “Phosphate” could result in receiving a hazardous raw material instead of a safe food additive.
FAQ
Is phosphate harmful to humans?
No. Phosphates are essential nutrients. However, industrial-grade phosphates must not be consumed. Only Food Grade Phosphates (like those produced by Goway Chemical) are purified to remove heavy metals and are safe for use in food processing within regulatory limits.
Does fertilizer contain phosphorus or phosphate?
Fertilizers contain Phosphates (like MAP, DAP, or SSP). However, on fertilizer labels, the nutrient content is often calculated and expressed as the percentage of Phosphorus Pentoxide (P₂O₅).
Looking for High-Purity Phosphates?
Goway Chemical manufactures Food Grade and Industrial Grade Sodium Phosphates using the high-purity thermal process.
