
Phosphate Manufacturer
GOWAY INTERNATIONAL MATERIAL CO., LTD has focused on Phosphate for over 10 years. Headquartered in Foshan National Torch Innovation and Pioneer Park, our factory in Sanshui Industrial Zone covers 60,000 square meters. We are the first automatic manufacturer of Sodium Tripolyphosphate (STPP) and STPP replacement in Guangdong, China. we have a single-line capacity of 30,000 tons.
We are mainly phosphate products, and the customers we serve are many from the food industry to the industrial industry. We are very familiar with the processing of phosphorus products in production. We have won the title of “National High-tech Enterprise”. We have our own laboratories and factories, and we are committed to providing customers with the highest quality services.
Goway Hot-Sale Phosphate
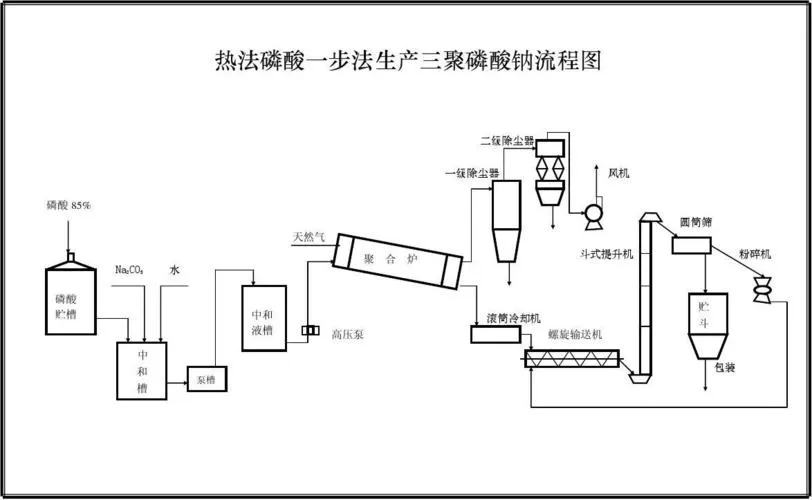
Phosphate Manufacturing Process
- Mining: The extraction of phosphate ore, typically from underground or open-pit mines, marks the initial step in the process. Phosphate rock and phosphate minerals are common ores.
- Crushing and Grinding: Crushing and grinding operations transform the mined ore into granules suitable for further processing.
- Leaching: The leaching process extracts phosphate from the ground ore by mixing it with acid, often sulfuric acid, to dissolve the phosphate.
- Concentration: Achieving a higher phosphate concentration involves concentrating phosphate from the leach solution, typically through precipitation or other methods.
- Crystallization and Precipitation: Extracting phosphate crystals from the concentrated solution is accomplished through crystallization and precipitation at this stage.
- Drying: To reduce moisture content and ensure suitability for various applications, the extracted phosphate crystals undergo a drying process.
- Blending and Granulation: Creating different types of phosphate products often requires blending them with other chemicals. Subsequently, the blended products are granulated to form suitable granules or pellets.
- Packaging and Distribution: The final phosphate products, meeting various specifications and sizes to fulfill customer requirements, undergo packaging before being distributed to customers.

Phosphate Benefits
Phosphate is a widely used chemical substance, which covers multiple fields.
Here are several main uses:
1. Agriculture: Phosphates can increase crop yields and quality while also improving soil quality. In agricultural production, phosphates are used as fertilizers to promote plant growth and development and improve plant disease resistance and stress resistance.
2. Food industry: Phosphates are used as food additives to improve the taste, color, and preservation of food. For example, phosphates can be used in making biscuits, bread, candies, and other foods to improve the shelf life and taste of the food.
3. Medical field: Phosphates are used as raw materials in pharmaceuticals to treat various diseases. For example, phosphates can be used to treat bone diseases such as fractures and osteoporosis and can promote bone growth and development.
4. Industrial field: Phosphates are used as catalysts, solvents, pigments, and other raw materials in the production of various chemicals. For example, phosphates can be used in the production of daily chemical products such as detergents and cosmetics to improve the quality and performance of the products.
5. Environmental governance: Phosphate can promote soil repair and water purification. When treating soil and water pollution, phosphates are used as additives or chemicals to promote the natural remediation process of soil and water.
Why Choose Us as Phosphate Supplier

Professional and Advanced Equipment
With more than ten years of experience in product manufacturing. Professional technicians provide you with professional advice. Advanced equipment creates high-quality products for you.

Low Price and High Quality
Under the same test conditions, the products of each batch are the same and do not affect the test results. The stability of product quality reduces the sample cost and time cost of customers.

Fast and Convenient Delivery
Fast production, early delivery, reliable logistics, can provide door-to-door service. Various transportation modes meet different countries.

Best After-sales Service Provided
Improve product sales and technical after-sales service. 24-hour online video call, actively solving problems for customers.








































